Face serums have become increasingly popular skincare products in Indonesia. With various brands and formulations available, many consumers are eager to try these products. However, before marketing a face serum, it’s important to understand the regulations and necessary steps, including obtaining a circulation permit (izin edar) from the Indonesian National Agency of Drug and Food Control (BPOM).
The Rise of Face Serums in Indonesia
In recent years, face serums have taken the Indonesian skincare market by storm, especially local products that offer specific benefits such as skin brightening, reducing dark spots, and increasing moisture.
Korean face serums, in particular, have become a major trend in Indonesia. Known for their innovative formulations, these serums offer a range of skin benefits. Popular ingredients in these serums include:
- Niacinamide: Known for brightening skin and reducing dark spots.
- Hyaluronic Acid: Helps maintain skin moisture.
- Centella Asiatica: Promotes skin healing and reduces irritation.
- Ginseng Extract and Snail Mucin: Offer anti-aging and hydration benefits.
One of the most popular trends is the “glass skin” concept, which refers to smooth, clear, and glowing skin. To achieve this look, using a serum with strong hydrating properties is highly recommended.

Before a face serum can be legally marketed in Indonesia, it must obtain a circulation permit from BPOM. This permit ensures that the product is safe for consumer use. The registration process involves testing and evaluation by BPOM to verify that the product meets safety and efficacy standards.
How to Register a Cosmetic Product in Indonesia
Where to Register Cosmetic Products? Cosmetic product registration is done through the Notifkos Online application, available at BPOM’s website: www.notifkos.pom.go.id.

Required Documents for Cosmetic Product Notification
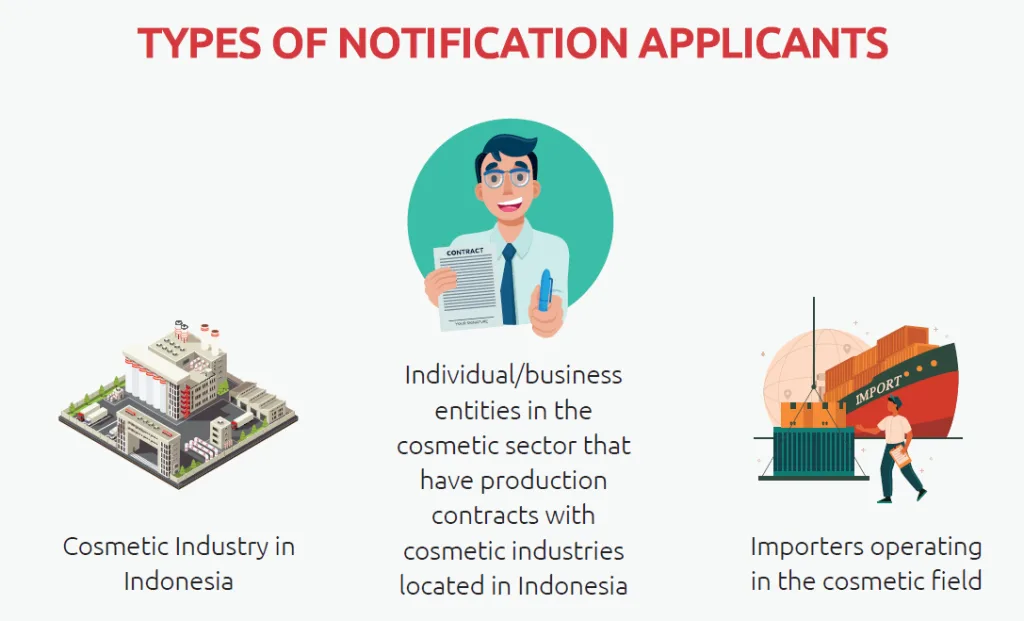
For Local Cosmetic Industry Applicants:
- NIB (Business Identification Number)
- Copy of ID (KTP) of Directors or Company Leaders
- Copy of Taxpayer Identification Number (NPWP)
- Copy of CPKB certificate (Good Manufacturing Practices for Cosmetics) valid for at least six months before expiry
- Statement letter from Directors/Leaders declaring no criminal involvement in the cosmetics field
- Trademark-related documents
For Importers:
- NIB
- Copy of ID (KTP) of Directors/Leaders
- Copy of Taxpayer Identification Number (NPWP)
- Letter of Authorization (LOA)
- Certificate for Sale (CFS)
- Good Manufacturing Practice (GMP)/ISO 22716
- Product Formula: Composition details, supplier identity, and functions of each ingredient.
- Product Presentation & Label: Outer/inner labels, packing specifications, and user instructions.
- Trademark-related documents
- Etc
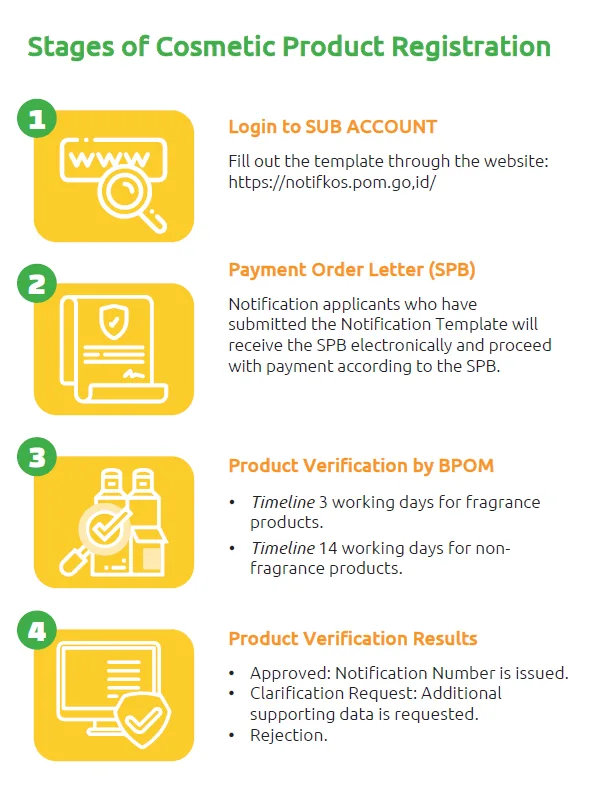
Steps to Register a Cosmetic Product
The registration process is carried out in two stages:
- Registration of the Notification Applicant (Business Entity Registration):
- For local cosmetic industries, required documents include NIB (Business Identification Number), a copy of the identity card of the company’s directors, NPWP (Tax Identification Number), and a CPKB (Good Manufacturing Practices) certificate or statement.
- For importers, additional documents such as a recommendation letter from BPOM, a copy of the agency appointment letter, and a Certificate of Free Sale (CFS) are required.
- Product Notification Registration:
- The applicant must fill out a business entity template on the Notifkos subsite, upload the required documents, and verify the documents at the local POM office.

How can INSIGHTOF help?
INSIGHTOF offers comprehensive regulatory services to facilitate market entry in Indonesia, specializing in BPOM and Kemenkes product registration with expert guidance every step of the way.
For any inquiries, please feel free to contact us at marketing@insightof.co.id.








