- Have any questions?
- 62-897-6470-070
- marketing@insightof.co.id
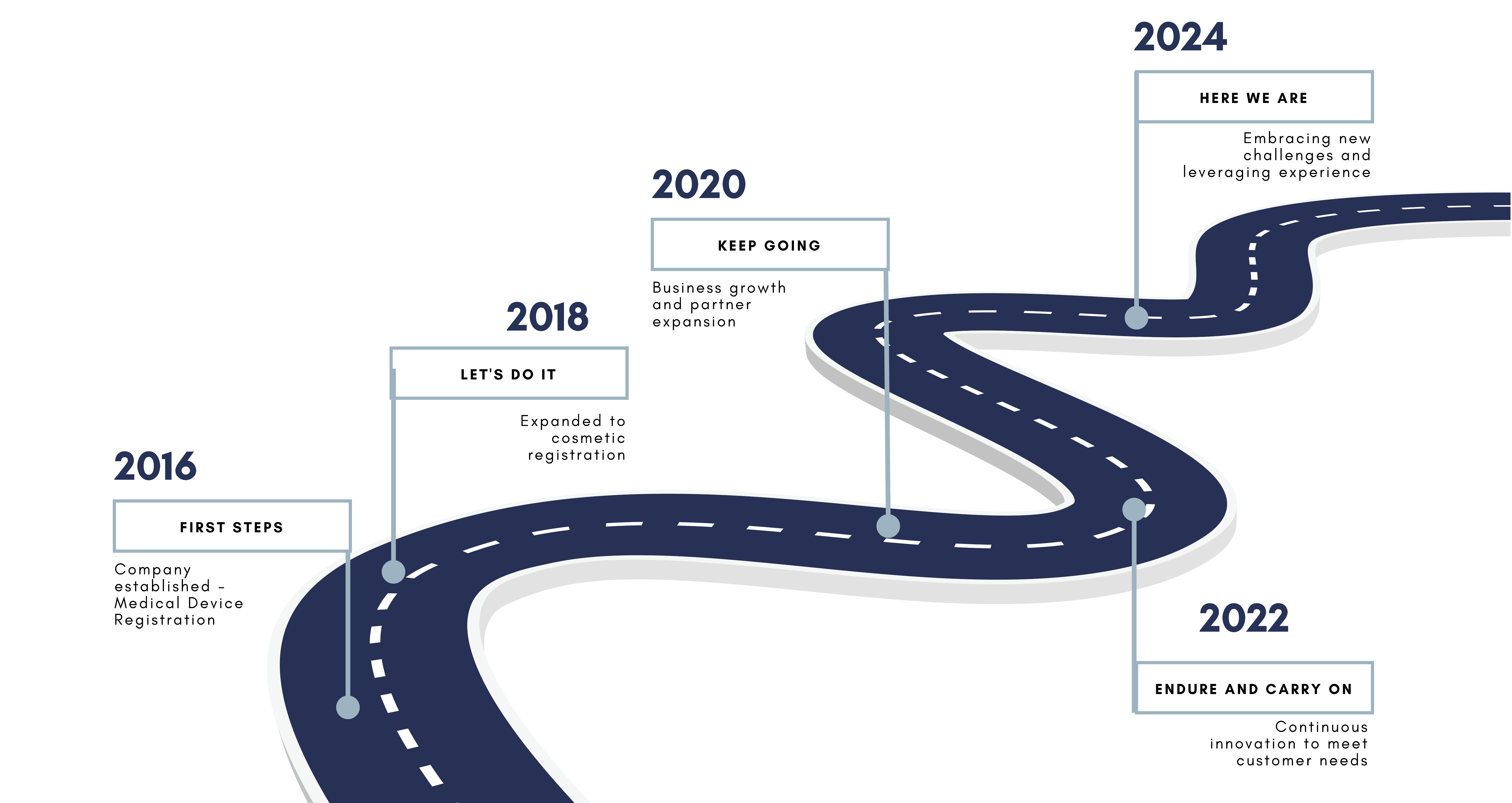
Since 2016, INSIGHTOF has been your trusted partner in navigating Indonesia’s regulatory landscape for medical devices and cosmetics. We specialize in product registration, certification, and permits, ensuring compliance with Kemenkes and BPOM standards.
Our mission is to provide unparalleled consulting services that enable our clients to achieve regulatory compliance efficiently and effectively. We are dedicated to ensuring that medical devices and cosmetics entering the Indonesian market meet all necessary standards, thereby safeguarding public health and promoting innovation in the industry.
Years Experienced
Manufactures/ Distributors
Product Registration


Our vision is to be the leading consulting firm in Indonesia for medical devices and cosmetics, recognized for our expertise, reliability, and commitment to excellence. We strive to foster a regulatory environment that supports the growth and success of our clients, ultimately contributing to the advancement of healthcare and beauty industries in Indonesia.
We uphold the highest ethical standards in everything we do.
We continuously seek new ways to our services and meet customer needs.
We believe in working closely with our clients and regulatory bodies to achieve success.
We are committed to maintaining and expanding our knowledge of regulatory requirements.
When a South Korean beauty brand approached us for assistance with their new cosmetic line, they faced tight deadlines and complex regulations. Our team conducted a thorough review of their product formulations and packaging, ensuring compliance with Kemenkes and BPOM regulations. We provided step-by-step guidance, which allowed the client to submit their registration applications promptly. Within just three months, we secured approvals, enabling the brand to launch on schedule and gain a competitive edge in the market.
A South Korean medical device company needed to register a new product in Indonesia but was unfamiliar with the local regulations. Recognizing the urgency, INSIGHTOF conducted a comprehensive regulatory analysis and developed a tailored strategy for the client. We liaised with Kemenkes to ensure all documentation was in order and addressed any questions that arose during the review process. Our proactive approach resulted in a swift approval, enabling the client to bring their innovative device to market quickly and efficiently.
A South Korean beauty products company aimed to enter the Indonesian market but struggled with the Indonesian Business Classification System (KBLI), risking compliance issues and registration delays. INSIGHTOF helped them accurately classify their products by conducting a thorough review and providing guidance on necessary documentation. As a result, the client successfully registered their products on time, ensuring compliance and facilitating a smooth market entry.

With our extensive experience in Indonesia’s regulatory environment, we provide the support you need to secure BPOM and Kemenkes approvals efficiently. Contact us today!
Wisma PMI 6th Floor Jl. Wijaya I No.63, RT.8/RW.1, Petogogan, Kec. Kby. Baru, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12160
marketing@insightof.co.id
Get The Latest Updates via email. Any time you may unsubscribe