- Have any questions?
- 62-897-6470-070
- marketing@insightof.co.id
Ensuring Compliance for Safe Medical Device Distribution
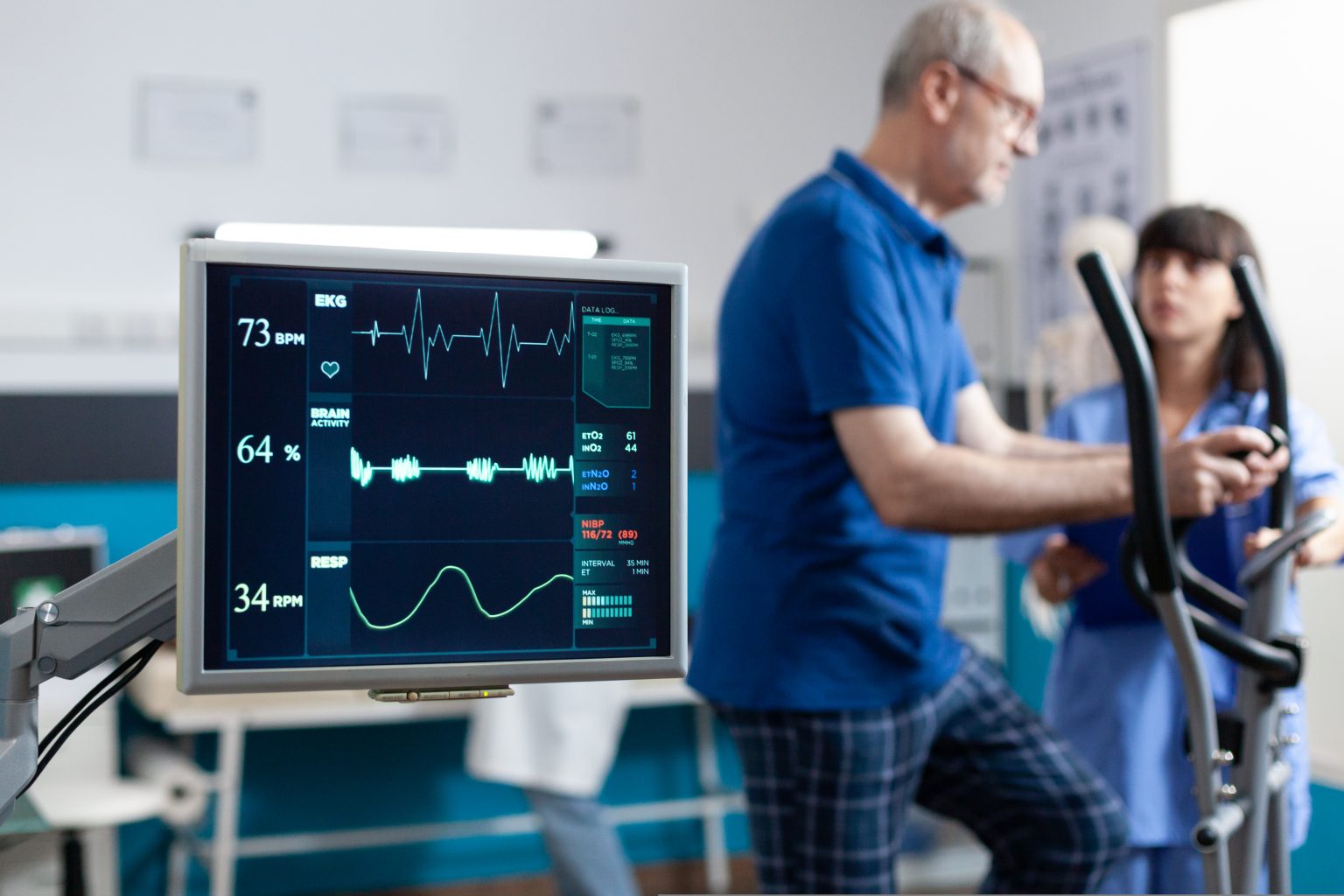
Contribute to the overall well-being of the Indonesian population by ensuring your products are safe and effective.
Medical Device Product Registration is the legal process required to ensure your medical devices meet the regulatory standards set by the Indonesian Ministry of Health. It ensures that only safe, effective, and compliant products are available for use in Indonesia.
Medical devices that use radiation for diagnosis or treatment, such as X-ray or radiation therapy machines.
Medical devices that use electrical energy but do not emit radiation, like ECG machines or ultrasound devices.
Medical devices that do not use electricity and are sterile, such as surgical instruments or implants.
Non-electric medical devices that are not sterile, including bandages and splints
Medical devices used to test samples outside the body, such as blood test kits or lab equipment.
Includes devices such as hospital beds and bandages.
Includes devices like infusion pumps and diagnostic equipment.
Includes devices such as ventilators and surgical lasers.
Includes devices like implantable pacemakers and defibrillators.
Slide Content
Slide Content
Slide Content
Slide Content
Slide Content
Slide Content
Slide Content
We’re here to help! Our team of experts provides clear guidance and support to ensure a seamless registration process, from understanding regulations to obtaining approval.
Wisma PMI 6th Floor Jl. Wijaya I No.63, RT.8/RW.1, Petogogan, Kec. Kby. Baru, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12160
marketing@insightof.co.id
Get The Latest Updates via email. Any time you may unsubscribe