Medical device distributors in Indonesia are required to comply with CDAKB guidelines to obtain or renew their distribution licenses (IDAK). Achieving this compliance often involves adhering to ISO 13485 standards as a key component of quality management practices. Manufacturers are urged to ensure their distributors meet these standards to uphold product quality and ensure regulatory compliance. But what exactly is ISO 13485?
What is ISO 13485?
ISO 13485 is an international standard specifically tailored for quality management systems (QMS) in the medical device industry. Originally published in 1996 and revised in 2003 and 2016, the most current version, ISO 13485:2016, provides a comprehensive framework for organizations involved in the entire lifecycle of medical devices, including design, development, production, storage, distribution, installation, and servicing.
Key Guidelines of ISO 13485
ISO 13485 encompasses several critical requirements to ensure the effectiveness of quality management systems in the medical device industry:
- Risk Management and Compliance: Establishes rigorous risk management practices throughout the device lifecycle, with a focus on compliance with relevant regulatory requirements.
- Design and Development: Specifies guidelines for controlling design and development activities to ensure that final products meet intended use and performance requirements.
- Production and Process Control: Emphasizes consistent quality through well-controlled manufacturing processes, including sterile device requirements.
- Supplier Management: Requires a systematic approach to managing suppliers, ensuring that purchased products and services meet specified requirements.
- Post-Market Surveillance: Involves monitoring devices after they are on the market to quickly address safety or performance issues.
Details of ISO 22716 Clauses
| Clause Number | Title | Product Description |
| Clause 1 | Scope | Defines where and to what extent the standard applies. |
| Clause 2 | Normative References | Lists references to related standards. |
| Clause 3 | Terms and Definitions | Provides definitions of key terms specific to ISO 13485. |
| Clause 4 | Quality Management System | Covers the documentation and structure of the QMS. |
| Clause 5 | Management Responsibility | Ensures top management is involved in maintaining the QMS. |
| Clause 6 | Resource Management | Ensures adequate resources, including personnel and infrastructure. |
| Clause 7 | Product Realization | Outlines requirements for product design, development, and production. |
| Clause 8 | Measurement, Analysis & Improvement | Focuses on QMS monitoring, measurement, and continual improvement. |
The first three sections are introductory, and the last five contain the mandatory requirements for the Quality Management System.
Benefits of Implementing ISO 13485
Adopting ISO 13485 offers numerous advantages for medical device manufacturers:
- Enhanced Product Safety: Ensures that medical devices meet regulatory requirements and are consistently safe for use.
- Market Access: Certification can facilitate market entry by demonstrating compliance with global regulatory standards, enhancing marketability.
- Improved Risk Management: The standard promotes a proactive approach to identifying and managing risks throughout the product lifecycle.
- Integration with Other Standards: ISO 13485 can be integrated with ISO 9001 and other quality management standards, streamlining quality control.
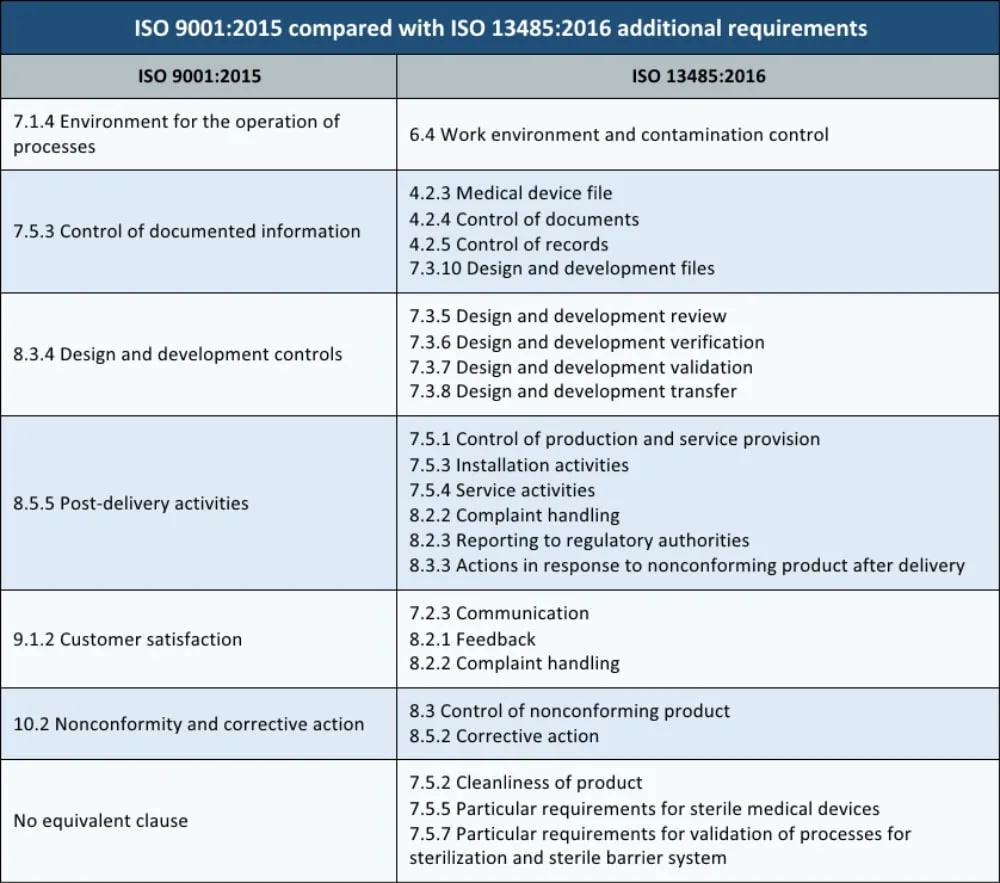
ISO 13485 vs ISO 9001
- Applicability: ISO 9001 is general and applicable to any organization, while ISO 13485 is specific to medical devices and related services.
- Risk Basis: ISO 9001 has shifted to a risk-based approach in recent versions, whereas ISO 13485 has always emphasized risk management.
- Additional Clauses in ISO 13485: ISO 13485 includes clauses not found in ISO 9001, such as those for design and development, sterilization, and software validation for medical devices.
- Certification Requirements: ISO 9001 certification is generally optional, while ISO 13485 certification is mandatory in Europe for selling medical devices.
INSIGHTOF, Your Partner in Medical Device Registration
At INSIGHTOF Consulting Indonesia, we specialize in guiding businesses through the regulatory requirements for medical device registration in Indonesia. While we do not provide ISO certification services, we offer essential support in the registration process, which may require ISO compliance. Our services include:
- Regulatory Compliance Guidance: We help businesses navigate the complexities of medical device regulations in Indonesia, including adherence to ISO standards.
- Documentation Support: Assistance in preparing required documents for regulatory submissions to ensure compliance with local requirements.
- Expert Consultation: Our team provides insights on best practices in quality management, helping companies establish effective systems for regulatory compliance.