As technology advances, software is increasingly integrated into healthcare, whether embedded in physical devices or functioning independently as standalone medical applications. In Indonesia, medical device software—although intangible—still falls under the regulatory scope of the Ministry of Health (Kementerian Kesehatan RI) and must follow a structured registration process.
But how does the registration process for software differ from conventional (hardware) medical devices?
What is Medical Device Software?
Medical Device Software refers to software applications designed to carry out medical functions, with or without physical components. This includes:
- Diagnostic apps
- Therapy management tools
- Medical image analysis software
- Health monitoring platforms
While software lacks a tangible form, it is still categorized as a medical device if it fulfills the definition and intended use criteria, especially under risk-based classifications.
In Indonesian regulation, this type of software is often called:
Perangkat Lunak sebagai Alat Kesehatan
(Software as a Medical Device / SaMD)
According to Kemenkes:
- Medical device software is software that meets the definition of a medical device.
- The software must be standalone software (not dependent on or embedded in other devices).
- Software validation is the result of testing to ensure that the software meets its technical specifications and requirements.
- Software used to design, manufacture, or operate another medical device (such as in vitro diagnostic equipment) is not considered standalone medical device software.
In other words, software that runs on general-purpose devices (like computers or phones) can still be registered as active medical devices, if they function independently for medical purposes. This interpretation aligns with ASEAN MDD and other international guidelines.
How is It Classified?
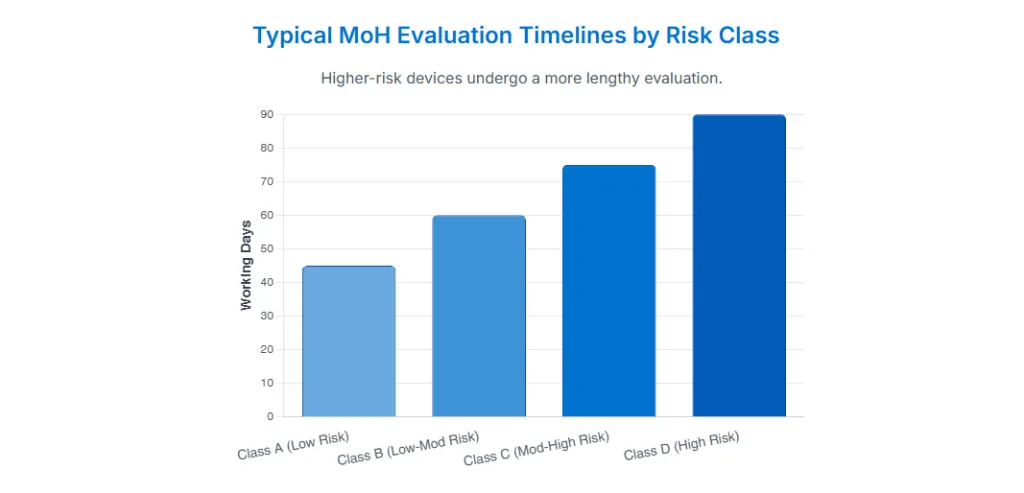
Under Permenkes No. 62/2017, standalone medical software is subject to the same four-tier risk classification system (Class A–D) used for hardware medical devices. However, the classification depends on the intended use or “cara penggunaan” of the software. Most Software as a Medical Device (SaMD) products fall into Class A or B, unless they are intended for high-risk or critical diagnostic/treatment purposes, in which case they may be classified as Class C or D.
Fundamental Prerequisites for Registration
Before a product can be registered, the license holder must meet two key regulatory requirements:
🔷 1. IDAK (Izin Distribusi Alat Kesehatan)
This is the Medical Device Distribution License, mandatory for all entities distributing medical devices in Indonesia. As of 2024, IDAK fully replaces the older IPAK license based on PP No. 5/2021 and Permenkes No. 14/2021.
IDAK licenses are valid for a period of five years and require renewal upon expiration, ensuring ongoing regulatory oversight. For foreign manufacturers, the typical pathway to fulfilling this requirement involves either establishing a local subsidiary or appointing a local distributor who already holds a valid IDAK.
🔷 2. CDAKB (Cara Distribusi Alat Kesehatan yang Baik)
In conjunction with the IDAK, Good Distribution Practice for Medical Devices (CDAKB), or Cara Distribusi Alat Kesehatan yang Baik, is a mandatory certification. CDAKB is equivalent to Good Medical Device Distribution Practices (GDP) and is issued by Indonesia’s Ministry of Health. Its primary purpose is to ensure the safe handling, storage, and transport of medical devices, thereby maintaining their quality control and ensuring optimal delivery to consumers.
All medical device distributors and their branches are legally required to implement CDAKB in all their distribution activities, as stipulated by Health Law No. 17 of 2023 and related regulations. Failure to comply with CDAKB can result in heavy penalties, as outlined in UU Kesehatan No. 17/2023.

Key Requirements for Medical Device Software Registration
Despite its intangible form, SaMD must meet all core regulatory and documentation requirements. Here’s how it differs from hardware registration:
IEC 62304 Certification (Mandatory)
According to Kemenkes:
“For medical device software, a certificate and test report for IEC 62304 must be provided, issued by an accredited testing laboratory or notified body.”
This standard verifies the software life cycle processes and ensures safety, reliability, and performance. It replaces the hardware requirement of IEC 60601 (electrical safety).
By contrast, software does not require:
- Electrical safety testing (IEC 60601)
- Biocompatibility testing
- Raw material specifications
- Packaging material specifications
Conformance with IEC 62304 typically requires a comprehensive set of documentation, often referred to as deliverables. These include a software development plan, software verification plan, software classification report, software description, software requirement specifications, software architecture document, software hazards analysis, cybersecurity plan, detailed design descriptions, a list of off-the-shelf software, code unit verification records, integration test reports, system software verification protocols, a summary test report, a trace matrix linking requirements to design and tests, revision level history, documentation of unresolved anomalies, and a defined software problem resolution process.
While Indonesian regulations may not explicitly mandate “IEC 62304 certification” as a standalone requirement, the explicit demand for “Software Verification and Validation Studies” and a “Software Test” strongly indicates that the underlying processes and documentation outlined in IEC 62304 are expected to demonstrate conformity. As IEC 62304 is a globally harmonized standard, adherence to its principles provides a recognized and robust framework for ensuring software quality and safety. This implies that manufacturers of medical device software must implement a rigorous software development lifecycle compliant with IEC 62304. This means integrating detailed planning, requirements management, design, implementation, and testing activities, along with comprehensive documentation, as a fundamental part of their overall medical device registration dossier for Indonesia. Simply stating that software is included will not suffice; detailed evidence of software quality and safety is required.
📦 No Packaging or Product Photo Required
Traditional devices require detailed material specifications and packaging information (e.g., package type, materials, labeling design). By contrast, software is intangible: there is no physical product to package or photograph. Indonesian registration tables show that packaging specifications (Spesifikasi kemasan) are required for all diagnostic devices, and raw-material specifications are required for Class C/D devices. These simply do not apply to software. Likewise, submitting photos of the product is not needed – instead, one might submit screenshots or mock-ups if helpful, but Indonesian authorities do not expect physical images of a software “device.”
For software:
- No packaging photos or box designs
- No raw material lists
📦 Clinical Trials mandatory for Class C and Class D
Clinical evidence is typically not required for software unless it is high-risk. In the MoH checklist, “Bukti klinis” (clinical evidence) is only mandated for Class C and D products. Most stand-alone software (e.g., simple diagnostic calculators, health-monitoring apps) fall into Class A/B and can be registered without clinical trial data. (If a software device makes critical clinical claims, local authorities may request literature or equivalence data, but routine Class A/B software is exempt from clinical testing.)
📝 IFU (Instructions for Use): A Critical Component
Because software has no physical label, there is no requirement to provide a sample label or packaging design as one would for hardware. However, clear instructions for use (IFU) in Indonesian are still required. The IFU must explain how to obtain, install, and operate the software. For example, it should include step-by-step guidance or a link for download, system requirements, login instructions, and how to interpret the output. A digital manual (PDF) is acceptable, but it must be “jelas dan mudah dimengerti” (clear and easy to understand). Unlike devices sold in boxes, there is no mandatory Kemenkes-wide packaging label, but the IFU itself must contain any regulatory marking (e.g., the assigned NIE number and manufacturer info) in Indonesian.
A well-written digital IFU in Bahasa Indonesia is essential. It must explain:
- Where and how to download the software
- System requirements and installation
- Setup or login instructions
- Operating instructions
- Troubleshooting steps
- Contact details and regulatory info (including NIE number)
Tip: The IFU replaces physical labeling and packaging for SaMD.
Registration Flow for Medical Device Software
- Obtain IDAK and CDAKB certification
- Prepare Required Dossier
- Submit Online via Regalkes (Kemenkes System)
- Wait for Approval and Receive Nomor Izin Edar (NIE) Number

Medical Device vs. Software Comparison Table
| Criteria | Physical Medical Device | Medical Device Software |
| Tangible Product | ✅ Yes | ❌ No |
| Product Photo | ✅ Required | ❌ Not required |
| Software Life Cycle (IEC 62304) | ❌ Not mandatory | ✅ Required |
| Packaging Specs | ✅ Required | ❌ Not applicable |
| User Manual | ✅ Required | ✅ Required |
Final Notes
In conclusion, registering medical device software in Indonesia generally follows the same regulatory structure as physical devices—but with specific adaptations for its non-physical nature.
By ensuring software meets all applicable technical and documentation standards, foreign developers and manufacturers can successfully bring their SaMD products to the Indonesian market.

Need Help Navigating Indonesian Registration?
If you’re developing or distributing medical software in Indonesia, partner with INSIGHTOF to ensure a smooth and compliant registration process—backed by deep local expertise and experience in Kemenkes regulations.
Do you need assistance registering your product in Indonesia?
Contact us today to start your registration process.



.svg/240px-YouTube_social_red_squircle_(2017).svg.png)






