Greetings to our respected Medical Device (Alkes) distributors,
In running a medical device distribution business, operational efficiency is key. One of the most frequently asked questions we receive—especially from clients who handle multiple imported products and local brands—is:
“Can a single storage warehouse be used to store different medical device brands?”
This confusion is understandable, considering that each brand—and even each product type—is tied to a different Distribution Permit and Medical Device Distributor License (IDAK).
The answer is: Yes, one warehouse can be used for multiple medical device brands, as long as you meet the strict requirements of Good Distribution Practice for Medical Devices (CDAKB).
At INSIGHTOF Consulting Indonesia, we have extensive experience helping companies optimize their storage facilities while remaining fully compliant with Ministry of Health (Kemenkes) regulations. This article explains how you can manage multiple brands under one roof with full regulatory certainty.

1. Regulatory Foundation: Efficiency and Compliance
The use of a single warehouse for multiple brands is a common and permitted practice under Indonesian regulations, provided quality and safety standards are met. The warehouse must be registered under your company’s Medical Device Distributor License (IDAK) and must hold a valid CDAKB (Good Distribution Practice for Medical Devices) certification.
The core requirement is your ability to demonstrate that products from different brands, classes, or storage requirements:
- Are not mixed and do not contaminate one another
- Are stored according to the specifications stated on each product’s label and product specification
- Have accurate and reliable distribution traceability records

2. Key Requirements for Multi-Brand Compliance in One Warehouse (CDAKB)
To ensure a single facility can legally and safely accommodate multiple medical device brands, you must focus on three main pillars of CDAKB compliance:
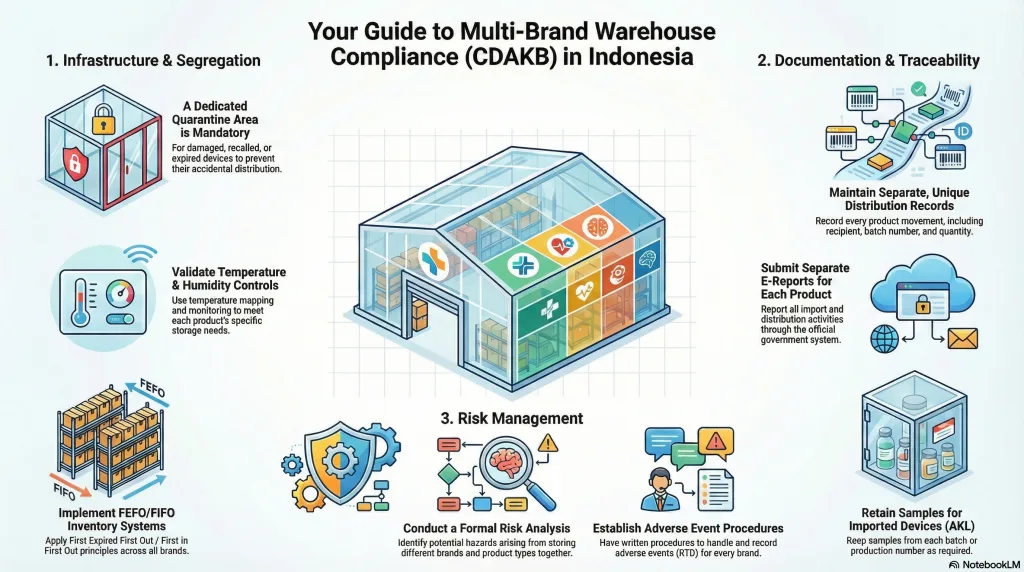
2.1. Infrastructure and Segregation
Physical and systematic segregation is the core of CDAKB in a multi-brand context.
| Technical Requirement | Warehouse Implementation | Compliance |
|---|---|---|
| Segregation of Damaged/Rejected Devices | A clearly marked quarantine area must be available for damaged, recalled, or expired medical devices. | Must guarantee that these products are not distributed. |
| Temperature & Humidity Control | Conduct regular temperature mapping to ensure storage temperatures comply with each product’s requirements (e.g., cold chain 2–8°C, room temperature 25°C). | Must have a validated monitoring system. |
| Separation of Imported vs. Local Products | Separate storage areas are recommended to simplify audits and import document traceability. | Must be distinguishable based on production origin (AKL or AKD). |
| Storage Arrangement | Apply FEFO (First Expired First Out) and FIFO (First In First Out) principles across all brands and batches. | Must use an integrated system such as WMS or stock cards. |
2.2. Documentation and Traceability
Even within one warehouse, each brand and product must maintain separate and unique records.
- Distribution Records: Every inbound and outbound movement of products must be recorded, including recipient name, batch number, and quantity.
- Retention Samples: Imported medical device distributors (AKL) must retain samples from each batch or production number, where applicable. Retention sample procedures must apply to all products, regardless of brand.
- E-Report Submission: Import and distribution activities must be reported separately for each product or brand through the official e-report system.
2.3. Risk Management
Using a single warehouse increases the risk of contamination and mis-shipment, especially for high-risk products (Class C and D).
- Adverse Event Procedures: Distributors must have written procedures and proper records for handling adverse events (Kejadian Tidak Diinginkan / KTD) for every brand distributed.
- Risk Management Analysis: A formal risk analysis must identify potential hazards arising from multi-brand storage, such as storing cold-chain products near non-temperature-sensitive devices.
3. Case Study and Compliance Recommendations
Common Distributor Issue: Distributor A represents three brands: Brand X (Cardiac Stents, Class D), Brand Y (Surgical Masks, Class B), and Brand Z (Ultrasound Devices, Class B). Their warehouse relies on standard air conditioning.
Analysis Outcome: Cardiac stents require strictly controlled temperature conditions. High-risk Class D products stored without validated temperature control were identified as a compliance risk.
INSIGHTOF Solution: INSIGHTOF assisted the distributor in developing a Temperature Mapping Protocol and Report to validate different temperature zones within the warehouse. Physical segregation was implemented by storing cardiac stents in temperature-controlled cabinets, allowing all brands to remain safely and compliantly stored in one warehouse.
4. FAQ: Brands, Warehouses, and Licensing
| Question | Answer |
|---|---|
| Do we need a new warehouse permit if we add one imported brand? | No. The same warehouse can be used, but the new product must be registered under the existing IDAK and evaluated for storage suitability. |
| Can one warehouse store medical devices and other products (e.g., cosmetics)? | Yes, but strict physical separation is required to prevent contamination and to comply with both CDAKB and Good Distribution Practice for Cosmetics (CDKB). |
| Who is responsible if a recall occurs for one brand stored in a shared warehouse? | The distributor (IDAK holder) is fully responsible for stopping distribution and conducting the recall. Proper traceability ensures only affected batches are recalled. |
| How long must records be retained for recalled products? | Import and distribution records must be retained for at least two years after distribution or for the product’s shelf life, whichever is longer. |
Conclusion
Using a single warehouse for multiple medical device brands is a smart efficiency strategy—but it requires layered compliance with CDAKB regulations. Success depends not on the number of warehouses, but on strong risk management, accurate documentation, and validated storage infrastructure.
INSIGHTOF Consulting Indonesia is your trusted partner to ensure your multi-brand warehouse remains compliant, audit-ready, and fully aligned with Kemenkes regulations.
Ready to optimize your warehouse for multiple medical device brands with confidence? Contact us today for a tailored compliance assessment.


#CDAKB #IDAK #MedicalDeviceWarehouse #SupplyChainManagement #MultiBrand #MedicalDeviceRegulation #Kemenkes #FEFO #FIFO #Traceability #IndonesiaRA #MedicalDeviceRegistration #CDAKBAudit








