If your cosmetic brand is planning to enter the Indonesian market with a unique formula, you’ll likely need to go through an ingredient evaluation process—especially if your product includes a new ingredient or an unfamiliar combination. So, how do you get a new cosmetic ingredient approved by BPOM (Indonesia’s National Agency of Drug and Food Control)?
In 2024, BPOM released an updated guideline that helps simplify—but also standardize—how new cosmetic ingredients are reviewed. Here’s what you need to know.
🌿 What Is Considered a “New” Ingredient?
A cosmetic ingredient is considered new in Indonesia if it:
- Is not listed in the NOTIFKOS database (Indonesia’s cosmetic ingredient system).
- Has a new INCI Name (even if the substance is similar to one already listed).
- Is part of a new combination of ingredients.
- Has a new function or usage claim.
- Is sourced using new extraction methods or formulations, like nanomaterials.

Step-by-Step: Submitting a New Cosmetic Ingredient for Approval
1. Prepare the Required Documents
Before applying for ingredient approval, BPOM requires you to compile several key documents, including:
- 📝 Formal Request Letter
- 🧾 Formulir Permohonan Kajian (Form A)
- ✅ Checklist of Data Completeness (Lampiran 6)
These documents must be submitted through BPOM’s online system, SIPK (Sistem Informasi Permohonan Kajian), at: https://standar-otskk.pom.go.id/sipk
2. Choose the Right Evaluation Path: Regular vs Fast Track
There are two evaluation tracks:
- Regular Track – Standard process that takes up to 85 working days
- Fast Track (Jalur Cepat) – Takes only 10 working days, but only certain ingredients qualify
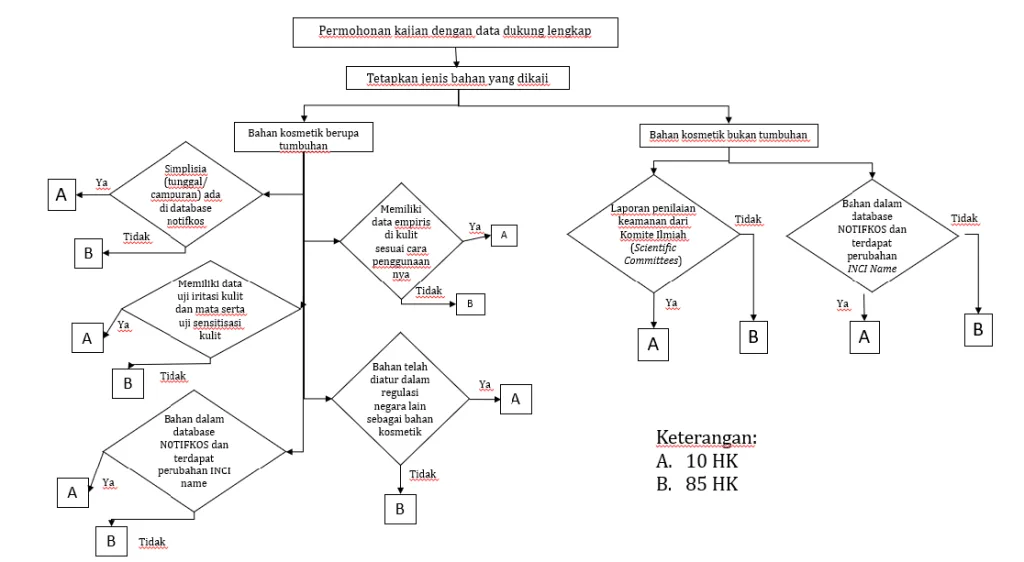
⚡ Fast Track Eligibility: Can Your Ingredient Qualify?
To use the Fast Track route, your cosmetic ingredient must meet specific criteria.
✅ For Plant-Based (Tumbuhan) Ingredients:
- Already listed in NOTIFKOS (even if used as a mix/simplisia)
- Has empirical safety data (e.g. traditionally used on skin)
- Has completed skin and eye irritation tests, and skin sensitization data
- May have new INCI Name, or is already regulated in other countries
Bonus tip: Include cosmetic safety data from existing global markets and any relevant cosmetovigilance data.
✅ For Non-Plant Ingredients:
- Comes with a safety assessment report from international scientific bodies such as:
- EU SCCS (Scientific Committee on Consumer Safety)
- US CIR (Cosmetic Ingredient Review)
- Has a change in INCI Name, but otherwise already known in NOTIFKOS
🔬 What Happens During the Evaluation?
BPOM evaluates both safety and quality of the ingredient. You’ll need to provide:
For Safety:
- Intended use and application
- Toxicological data (e.g. irritation, sensitization, absorption)
- Published studies or scientific opinions (e.g. SCCS, CIR, ACSB)
- Cosmetovigilance (adverse reaction) reports if applicable
For Quality:
- Certificate of Analysis (COA)
- Ingredient origin and manufacturing method
- Purity specifications
- Analytical testing methods
If your ingredient is a nanomaterial, additional data such as absorption and nano-specific safety studies are required.
🏭 Special Notes for Local Manufacturers
If you’re manufacturing under Golongan B (Class B) in Indonesia (typically small-scale or simpler tech manufacturers), the BPOM also assesses:
- Your production facility’s capability
- Whether the ingredient’s risk level matches the level of your production technology
Do you need assistance registering your product in Indonesia?
Contact us today to start your registration process.
Contact us or message us via WhatsApp: wa.me/62897647007
Sources








