1. Health Supplement Market in Indonesia
Indonesia has experienced a rapid increase in health awareness, particularly in the post-pandemic era. Demand for vitamins, minerals, immunity boosters, and herbal supplements continues to grow significantly. However, entering this promising market requires strict compliance with regulations enforced by the National Agency of Drug and Food Control (BPOM – Badan Pengawas Obat dan Makanan).

Unlike general food products, health supplements are regulated based on their functional benefits and active ingredients. BPOM conducts comprehensive evaluations to ensure product safety, efficacy, and quality before approval. Understanding and navigating this regulatory framework is essential for both local and international companies seeking to operate legally in Indonesia.
2. About INSIGHTOF Consulting Indonesia
INSIGHTOF Consulting Indonesia specializes in bridging innovative health products with Indonesian regulatory compliance. With extensive experience in BPOM registrations, we assist manufacturers and distributors in obtaining Marketing Authorization (Izin Edar) efficiently.
From dossier preparation to final approval, our team ensures a smooth, compliant, and commercially viable registration process.

3. Understanding Health Supplement Regulations
Under BPOM Regulation No. 32 of 2022, health supplements are defined as products intended to supplement nutritional intake and help maintain, improve, or enhance health functions. These products may contain vitamins, minerals, amino acids, or other non-plant-derived ingredients with nutritional or physiological effects.
Health supplement is a product intended to supplement nutritional needs; maintain, improve and / or improve health functions; having nutritional value and / or physiological effects; containing one or more ingredients in the form of vitamins, minerals, amino acids and / or other non-plant ingredients can be combined with plants.
Key Regulatory Points:
- Regulatory Authority: BPOM
- Registration Platform: ASROT (Aplikasi Sistem Registrasi Obat Tradisional dan Suplemen Kesehatan)
- Mandatory Requirement: All health supplements—locally produced or imported—must obtain a Distribution Permit Number (NIE – Nomor Izin Edar) before commercialization.
- NIE Code Prefixes:
- SD – Locally Manufactured Health Supplements
- SI – Imported Health Supplements
- SL – Licensed Health Supplements (Manufactured Locally under License)
POM SD/SI/SL 123456789


Who Is Allowed to Register Health Supplements in Indonesia?
According to BPOM regulations, the following entities are authorized to register health supplements in Indonesia:
- Pharmaceutical Industries
Companies holding a valid pharmaceutical manufacturing license in Indonesia. - Traditional Medicine Industries, including:
a. Traditional Medicine Industry
b. Small-Scale Traditional Medicine Business
c. Micro-Scale Traditional Medicine Business - Food Industries
Licensed food manufacturers that meet BPOM requirements for producing health supplement products. - Importers Engaged in Health Supplement Marketing
Indonesian-registered companies authorized to import and distribute health supplements, acting as the local license holder for imported products.
KBLI Classification for Health Supplement Business Activities (SK)
In Indonesia, business eligibility for health supplement registration is closely linked to the Indonesian Standard Industrial Classification (KBLI 2020).
Examples of Relevant KBLI Codes
For Manufacturers:
- 21012 – Pharmaceutical Product Industry for Human Use
- 21022 – Traditional Medicine Industry for Human Use
- 11040 – Soft Drink Industry
- 11090 – Other Beverage Industry
- 10799 – Other Food Product Industry
For Importers / Distributors:
- 46441 – Wholesale Trade of Pharmaceutical Products for Human Use
- 46442 – Wholesale Trade of Traditional Medicines for Human Use
- 46334 – Wholesale Trade of Non-Alcoholic Beverages (Non-Dairy)
- 46339 – Wholesale Trade of Other Food and Beverages
4. Prerequisites for Registration
Before initiating the registration process, companies must fulfill certain administrative and facility-related requirements.
The following lists are provided as examples only and may vary depending on the product type, risk classification, and BPOM evaluation.
4.1 Local Manufacturers
- Single Business Number (Nomor Induk Berusaha, NIB)
- Certificate of CPOB / CPOTB / CPPOB / Certificate of partial CPOTB
- Individual Tax Number (Nomor Pokok Wajib Pajak, NPWP)
- Notarial deed of the company
- Letter of authorization stating person in charge of company account
4.2 Importers / Distributors
- NIB with relevant KBLI codes for pharmaceutical or traditional product trading
- Letter of Authorization (LoA) from the overseas manufacturer
- Certificate of Free Sale (CFS) issued by the competent authority
- Notarial deed of the company
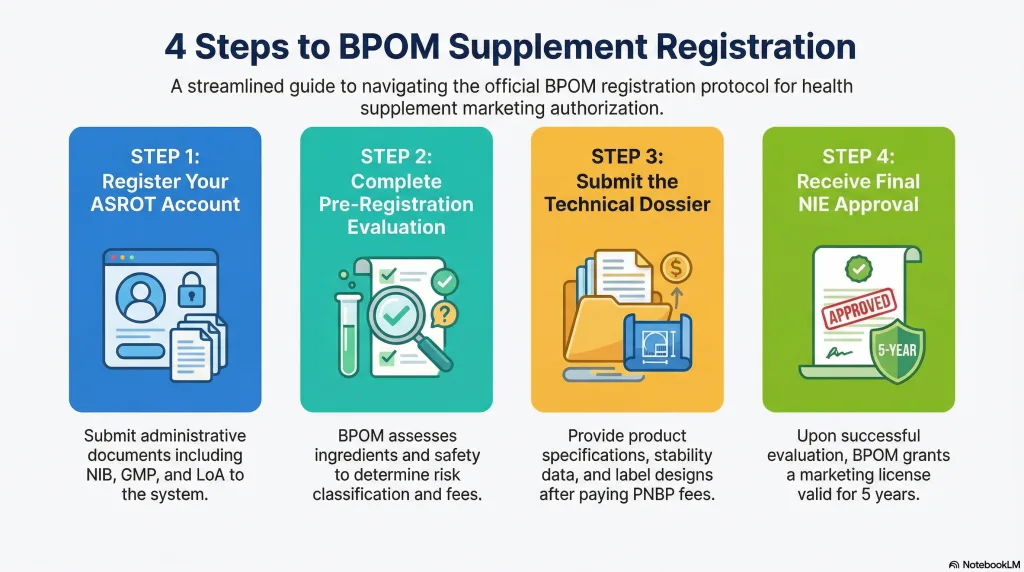
5. Health Supplement Registration Process (Step-by-Step)
Phase 1: Account Registration
The company must register in BPOM’s ASROT system by submitting administrative documents such as NIB, GMP, and LoA.
Phase 2: Pre-Registration
BPOM evaluates:
- Product formulation and ingredients
- Manufacturing process
- Safety and efficacy data
This phase determines the risk classification (Low Risk or High Risk) and applicable registration fees.
Phase 3: Registration (Dossier Submission)
After pre-registration approval and payment of PNBP (Non-Tax State Revenue) fees, the applicant submits a complete technical dossier, including:
- Finished product specifications
- Testing methods
- Certificates of Analysis (CoA)
- Stability study data
- Label and packaging design
Phase 4: Evaluation and Approval
BPOM conducts a detailed evaluation. If necessary, additional data requests (Tambahan Data) will be issued. Upon full compliance, BPOM grants the NIE, valid for 5 years.
6. Common Challenges in Supplement Registration
- Prohibited or Restricted Ingredients: Some ingredients that are permitted in other countries may be prohibited or restricted in Indonesia. All formulations must comply with BPOM’s negative and restricted ingredient lists.
- Stability Studies: BPOM may require stability study data conducted under Zone IVb climatic conditions (30°C ± 2°C / 75% RH ± 5%), particularly for imported products. Stability data from other zones may be subject to rejection or require additional justification.
- Labeling Claims: All claims must be scientifically substantiated and aligned with the approved function of health supplements. Therapeutic or disease-curing claims are strictly prohibited.
- Animal-Derived Ingredient: Any animal-derived ingredients must be clearly declared, including their source. Supporting documentation may be required, especially for halal compliance.
7. Halal Certification for Health Supplements
After obtaining the Marketing Authorization (NIE), companies must prepare for Halal Certification. Under Indonesia’s Halal Product Assurance Law (Jaminan Produk Halal – JPH), health supplements are subject to mandatory halal certification with a phased implementation deadline.
Based on Government Regulation No. 42 of 2024 on the Implementation of Halal Product Assurance, the mandatory halal certification timelines for medicinal and health-related products are regulated as follows:
- Natural medicines, quasi-drugs, and health supplements
Mandatory halal certification applies from 17 October 2021 until 17 October 2026. - Over-the-counter (OTC) medicines and limited OTC medicines
Mandatory halal certification applies from 17 October 2021 until 17 October 2029. - Prescription medicines (excluding psychotropic drugs)
Mandatory halal certification applies from 17 October 2021 until 17 October 2034.
For health supplements, this means that all products must be halal-certified no later than 17 October 2026 to remain legally marketable in Indonesia. Early preparation is strongly recommended to avoid market disruption, reformulation risks, or distribution restrictions.

8. Frequently Asked Questions (FAQ)
Q: Can foreign companies register supplements directly in Indonesia?
A: No. Foreign companies must appoint a local Indonesian entity as the license holder.
Q: How long is the NIE valid?
A: Five (5) years, renewable.
Q: Can products be sold during registration?
A: No. Selling without a valid NIE is illegal and subject to sanctions.
Q: Can dietary supplement advertisements claim to cure diseases?
A: Based on the Decree of the Head of the National Agency of Drug and Food Control (BPOM) No. HK.00.05.23.3644 of 2004 concerning the Basic Provisions for the Supervision of Dietary Supplements, dietary supplements are products intended to supplement nutritional intake. Therefore, dietary supplement products are not allowed to claim the treatment or cure of diseases.
How INSIGHTOF Consulting Indonesia Can Support You
INSIGHTOF Consulting Indonesia provides end-to-end regulatory support to ensure your products enter the Indonesian market smoothly and compliantly.
Our Services Include:
✔ Ingredient and formula gap analysis
✔ Administrative document verification
✔ Full dossier preparation and submission

Conclusion
Registering health supplements in Indonesia requires careful compliance with BPOM regulations covering ingredients, stability studies, labeling, and documentation. With thorough preparation and expert guidance, businesses can avoid costly delays and secure faster market entry.
Ready to launch your health supplement in Indonesia?
INSIGHTOF Consulting Indonesia provides the regulatory expertise you need to obtain BPOM approval efficiently. Contact us today to start your consultation.

#BPOMRegistration #HealthSupplementIndonesia #IzinEdarBPOM #DietarySupplement #HalalSupplement #RegulatoryCompliance #BPOMConsultant #INSIGHTOFConsulting #SupplementRegistration #IndonesianMarketEntry








