For businesses that intend to distribute cosmetic products in Indonesia, possessing a distribution permit or Cosmetic Notification from the National Agency of Drug and Food Control (BPOM) is an absolute requirement. However, before registering their products, certain types of companies must first secure a crucial document called the Cosmetic Notification Recommendation.
What is the Cosmetic Notification Recommendation?
The Cosmetic Notification Recommendation is an official letter issued by the local BPOM Technical Implementation Unit (UPT BPOM) (Balai Besar/Balai/Loka POM) which certifies that a business entity has met the administrative and technical requirements to act as a notification applicant.

This document is mandatory for two categories of business players:
- Cosmetic Importers: Companies that import finished cosmetic products from abroad.
- Contract Manufacturing Providers (Maklon): Individuals or business entities that own a brand but outsource the production of their cosmetics to a manufacturing facility in Indonesia (a third party).
The Cosmetic Notification Recommendation is the initial gateway. Without this recommendation, importers or contract providers cannot log into the BPOM e-Notification system to register their products.
This same principle applies if you want to distribute drugs, food and beverages in Indonesia.


The Role of the PSB Audit
The process of obtaining an Cosmetic Notification Recommendation cannot be separated from the activity known as the Balai Inspection (PSB). PSB is a physical inspection activity carried out by BPOM officials on site.

PSB is More Than Just a Physical Check
The purpose of the PSB is to directly verify the eligibility of the facilities owned by the RSPN applicant, namely the office and storage warehouse. This inspection goes beyond just viewing the physical condition; it ensures that the company implements aspects of Good Cosmetic Distribution Practices (CDKB).
The main aspects scrutinized during a PSB include:
- Physical Facilities: The condition of the warehouse, sanitation, and control of temperature and humidity to ensure product quality is maintained.
- Personnel: The qualification and presence of a Technical Responsible Person (PJT) with appropriate educational background (Pharmacy, Chemistry, Biology, or Medicine).
- Documentation and Procedures: The completeness and readiness of key documents, such as:
- Written procedures for the procurement, reception, storage, and release of cosmetics.
- Inventory tracking system (stock cards).
- Procedures for handling complaints and product recalls.
The Flow of Interrelation
Simply put, the flow for obtaining an Cosmetic Notification Recommendation is:
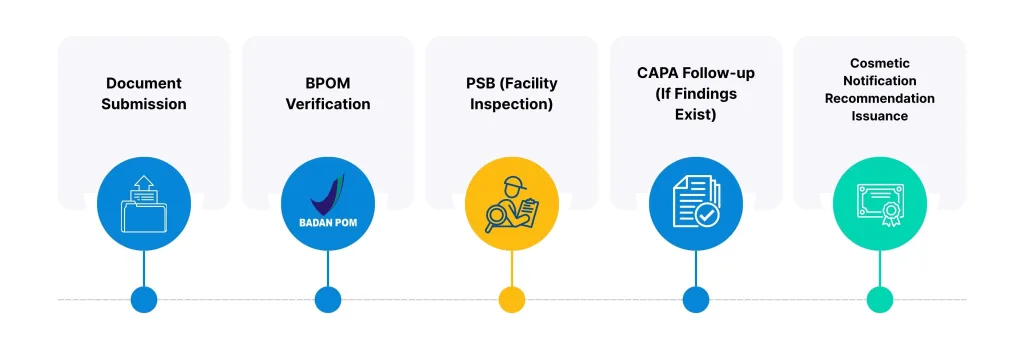
If the PSB results show any deficiencies or non-compliance (TMK/Tidak Memenuhi Ketentuan), the applicant will be given time to make corrections and submit a Corrective and Preventive Action (CAPA) report. The Recommendation will only be issued after BPOM approves the CAPA report.
Therefore, it can be concluded that PSB is the mandatory on-site verification process that forms the legal basis for the issuance of the Cosmetic Notification Recommendation. The Cosmetic Notification Recommendation is the final outcome, and the PSB is the vital step that must be completed.
INSIGHTOF Consulting Indonesia can help you obtain your Cosmetic Notification Recommendation, including full support throughout the PSB audit process to ensure compliance and smooth approval.
📩 Contact us today and start your registration process with confidence.
Do you need assistance registering your product in Indonesia?
Contact us today to start your registration process.




.svg/240px-YouTube_social_red_squircle_(2017).svg.png)






