1. Introduction
Navigating the regulatory landscape for medical devices in Indonesia has become increasingly stringent in recent years. Many distributors and manufacturers are surprised to find their product registration applications rejected or delayed because they lack a specific certification known as CDAKB. In previous years, holding a distribution license (IDAK) was sufficient. However, the Indonesian Ministry of Health (Kemenkes) now strictly enforces the implementation of Good Distribution Practice standards to ensure product safety from the warehouse to the patient. Understanding and obtaining CDAKB is no longer optional; it is a critical requirement for business continuity.
2. About INSIGHTOF Consulting
We are INSIGHTOF Consulting Indonesia, a dedicated regulatory affairs firm specializing in Medical Devices, Cosmetics, Food, and Halal certification. We understand that the shift from simple licensing to comprehensive quality assurance systems can be overwhelming for businesses. Our team provides end-to-end assistance, from initial gap analysis to the final issuance of your CDAKB certificate, ensuring your operations remain compliant and your market access uninterrupted.
3. Understanding CDAKB: A Comprehensive Guide
3.1 What is CDAKB?
CDAKB stands for Cara Distribusi Alat Kesehatan yang Baik, which translates to Good Distribution Practice for Medical Devices. It is a certification system established by the Indonesian Ministry of Health to ensure that medical devices are procured, stored, handled, and distributed in a way that maintains their quality, safety, and performance.
While the distribution license (IDAK) grants you the legal right to operate as a distributor, the CDAKB certificate proves that your operational procedures adhere to international quality standards. It covers every aspect of the supply chain, including personnel competency, warehouse facilities, cold chain management, and post-market surveillance.

3.2 Why CDAKB is Now Mandatory
Previously, CDAKB was a voluntary standard. However, recent regulatory updates have integrated CDAKB into the requirements for obtaining and renewing Marketing Authorizations (Izin Edar).
- Prerequisite for Product Registration: The online registration system (REGALKES) often requires proof of CDAKB implementation before allowing new product submissions.
- License Renewal: Distributors cannot renew their IDAK (Distribution License) without a valid CDAKB certificate.
- Quality Assurance: It ensures that sensitive devices, such as IVD reagents or electromechanical equipment, are not damaged during storage or transport.
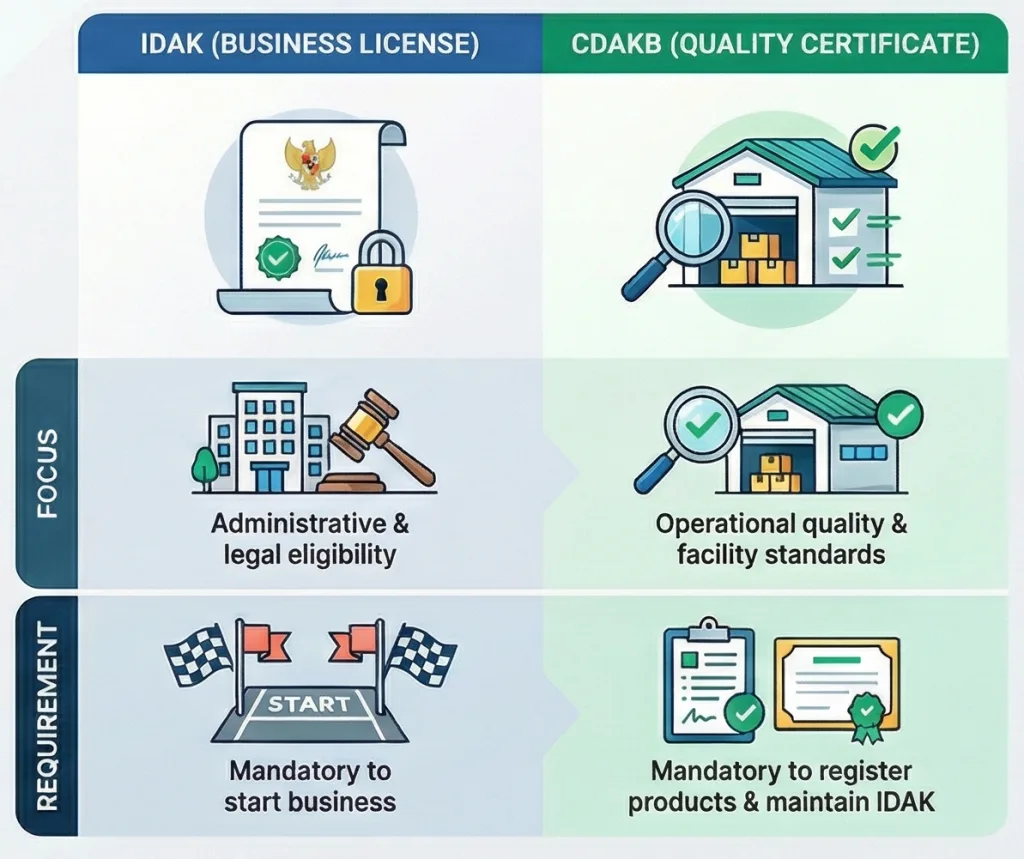
3.3 IDAK vs. CDAKB: What is the Difference?
It is crucial to distinguish between the business license and the quality certification. Please refer to the table below for a clear comparison.
| Feature | IDAK (Izin Distribusi Alat Kesehatan) | CDAKB (Cara Distribusi Alat Kesehatan yang Baik) |
| Definition | A business license authorizing a company to distribute medical devices. | A certificate attesting to the implementation of a Quality Management System in distribution. |
| Focus | Administrative and legal eligibility. | Operational quality, SOPs, and facility standards. |
| Audit Type | Administrative review and basic site visit. | Rigorous on-site audit of processes, records, and facilities. |
| Validity | 5 Years. | 5 Years (subject to surveillance audits). |
| Requirement | Mandatory to start business. | Mandatory to register products and maintain IDAK. |
3.4 Key Aspects of the CDAKB Audit
To obtain certification, your company must undergo an audit by Kemenkes officials. They will inspect several critical aspects:
- Management Responsibility: Commitment from top management to maintain quality.
- Resource Management: Competency of the Technical Responsible Person (PJT) and staff training records.
- Supply Chain Activities: SOPs for procurement, receiving, storage, and delivery.
- Traceability: Ability to track products from manufacturer to end-user, especially for recalls.
- Facility Management: Temperature monitoring logs, pest control, and cleanliness.
- FSCA (Field Safety Corrective Action): Procedures for handling complaints and recalls.
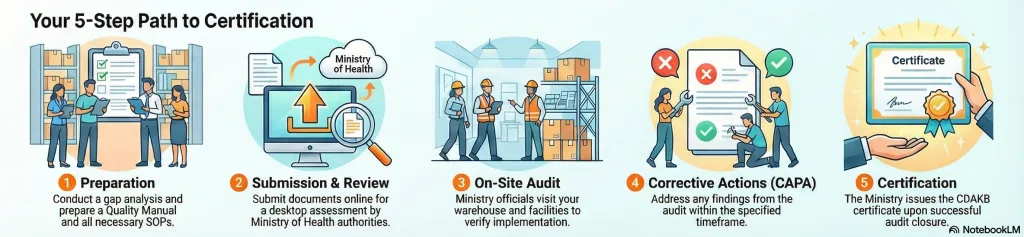
3.5 The Certification Process Steps
The path to CDAKB involves several stages that require careful preparation:
- Gap Analysis: Assess current facilities and SOPs against CDAKB standards.
- Document Preparation: Create a Quality Manual and detailed Standard Operating Procedures (SOPs).
- Online Submission: Submit the application via the Ministry of Health’s portal.
- Desktop Assessment: Evaluators review the submitted documents.
- On-Site Audit: Auditors visit the warehouse to verify implementation.
- CAPA (Corrective Action and Preventive Action): Address any findings from the audit within a specific timeframe.
- Issuance: The Ministry issues the CDAKB certificate upon successful CAPA closure.
4. Frequently Asked Questions (FAQ)
Q: My company is a foreign manufacturer. Do I need CDAKB?
A: No, CDAKB applies to the Indonesian entity distributing the product. However, your local distributor or license holder in Indonesia must possess this certification.
Q: Can we apply for product registration (Izin Edar) if our CDAKB is still in process?
A: In some cases, a receipt of CDAKB submission may be accepted temporarily, but having the full certificate is necessary for long-term compliance and smooth renewal processes.
Q: What happens if we fail the audit?
A: You will receive a list of findings (minor, major, or critical). You must implement corrective actions (CAPA) and submit evidence of these corrections. If critical findings are not addressed, certification will be denied.
Q: Does CDAKB apply to all risk classes of medical devices?
A: Yes, it applies to all distributors, but the complexity of the requirements scales with the risk of the products (e.g., cold chain requirements for IVD reagents are stricter than for hospital furniture).
Achieve CDAKB Compliance with INSIGHTOF

Preparing for a Ministry of Health audit can be stressful without proper guidance. INSIGHTOF Consulting Indonesia offers a comprehensive support package to ensure you pass your CDAKB audit with confidence.
![]() SOP Development: We assist in drafting and refining your Quality Manual and Standard Operating Procedures to meet regulatory standards.
SOP Development: We assist in drafting and refining your Quality Manual and Standard Operating Procedures to meet regulatory standards.
![]() Mock Audits: Our experts conduct simulated audits to identify non-compliance issues before the official Kemenkes inspectors arrive.
Mock Audits: Our experts conduct simulated audits to identify non-compliance issues before the official Kemenkes inspectors arrive.
![]() PJT Training: We guide your Technical Responsible Person (PJT) on how to answer auditor questions effectively.
PJT Training: We guide your Technical Responsible Person (PJT) on how to answer auditor questions effectively.
![]() CAPA Assistance: If findings occur, we help formulate and document the necessary corrective actions to close the audit successfully.
CAPA Assistance: If findings occur, we help formulate and document the necessary corrective actions to close the audit successfully.
Conclusion
CDAKB is no longer just a badge of excellence; it is a fundamental license to operate in the Indonesian medical device market. Ignoring this requirement can lead to halted product registrations and an inability to renew distribution licenses. By establishing a robust quality management system now, you safeguard your business against regulatory disruptions and demonstrate your commitment to patient safety. Partner with INSIGHTOF Consulting to navigate this complex process efficiently.
If you need expert assistance with your CDAKB certification or medical device registration, please contact us for a consultation.
#CDAKB #GoodDistributionPractice #MedicalDeviceIndonesia #IzinEdarAlkes #Kemenkes #AlatKesehatan #RegulatoryAffairsIndonesia #MedicalDeviceDistributor #IDAK #QualityManagementSystem #IndonesianHealthRegulation #MedicalDeviceConsultant #ISO13485 #GDP #INSIGHTOF







