INSIGHTOF has supported cosmetic, medical device, and health product companies in Indonesia since 2016. With more than 1,000 BPOM, Halal, and medical device registrations processed, our team helps ensure that products comply fully with Indonesian regulatory requirements.
10 Years Experience / Certification Consulting Expert / Based in Jakarta
During recent consultations, many cosmetic companies have raised similar questions:
- “We have sold whitening or anti-aging products for years. Why is BPOM now asking about clinical trials?”
- “Do we really need ethics approval just to test a cosmetic product?”
These concerns are understandable.
With the issuance of BPOM Regulation No. 34 of 2025, BPOM formally strengthens the framework for cosmetic clinical trials, especially for products with specific claims, innovative ingredients, or higher safety risk.
The regulation reflects BPOM’s commitment to science evidence-based evaluation, ensuring that cosmetic claims related to safety and benefits are scientifically valid and ethically conducted.
If supporting data in your Product Information Document (DIP) is insufficient or non-compliant, the notification process may be delayed or questioned during audit or post-market surveillance.
Ethics Approval: When Is It Mandatory?
One of the most important clarifications under this regulation relates to ethics approval.
If a new clinical trial is conducted in Indonesia, then ethics approval is mandatory before the trial begins. This requirement applies to both local and imported products. For high-risk products—such as cosmetics containing specific whitening agents, nano ingredients, anti-aging claims, SPF claims, or products applied to sensitive areas—approval must be obtained from an independent Ethics Committee.
Existing overseas ethics approval cannot replace Indonesian ethics approval for a new clinical trial conducted in Indonesia.
Can Imported Products Use Existing Overseas Clinical Trial Data?
This does not mean that all imported products must repeat clinical trials in Indonesia.
For imported cosmetic products, including those from Korea, existing overseas clinical trial data can still be used if all of the following conditions are met:
- The clinical trial has proper ethical approval
- The Ethics Committee is clearly identified
- Informed consent was obtained from all subjects
- The methodology is scientifically valid
- The claims used in Indonesia are the same as, or more conservative than, the claims supported by the clinical trial
In this situation, the existing clinical trial data can be used, and no new ethics approval or repeat clinical trial in Indonesia is required.
However, problems arise when the existing clinical trial does not support the intended claim. For example, if a serum only has clinical data supporting hydration, but the brand plans to use whitening or anti-aging claims in Indonesia, the data is considered insufficient. In such cases, there are only two compliant options: either adjust the claim to match the available data, or conduct a new clinical trial. If a new clinical trial is conducted in Indonesia, ethics approval must be obtained before the trial starts.
Does a New Clinical Trial Always Need Indonesian Ethics Approval?
👉 YES, if the trial is conducted in Indonesia.
Under BPOM Regulation No. 34 of 2025:
- All clinical trials conducted in Indonesia must obtain ethics approval BEFORE starting
- This applies to local and imported products
- High-risk products (e.g. whitening agents, nano ingredients, anti-aging claims) require independent Ethics Committee approval
Existing overseas ethics approval cannot replace Indonesian ethics approval for a new trial.
 Regulatory Updates in BPOM Regulation No. 34 of 2025
Regulatory Updates in BPOM Regulation No. 34 of 2025

BPOM Regulation No. 34 of 2025 amends BPOM Regulation No. 8 of 2024 on Clinical Trial Approval Procedures (PPUK) by introducing specific, binding provisions for cosmetic clinical trials.
A new provision, Article 5 paragraph (3a), explicitly states that cosmetic clinical trials must follow the Cosmetic Clinical Trial Guidelines listed in Annex IIIa, which form an inseparable part of the regulation. Cosmetic trials are no longer guided by informal practices. Methodology must be valid, published, scientifically justified, and ethically sound, with subject safety as the top priority.
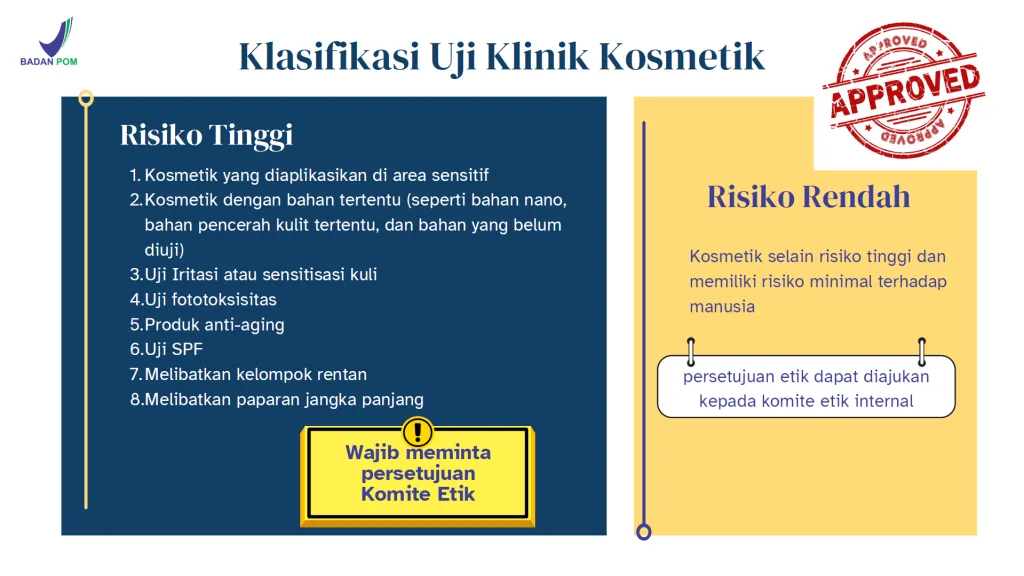
The regulation also introduces a risk-based classification of cosmetic clinical trials. High-risk products include anti-aging products, SPF products, cosmetics with nano materials or specific whitening agents, products applied to sensitive areas, irritation or sensitization tests, phototoxicity tests, and products involving vulnerable groups or long-term exposure. Low-risk products generally include basic moisturizers, cleansers, and products with well-established ingredients and minimal physiological impact.
For high-risk trials, approval from an independent Ethics Committee is mandatory. For low-risk trials, ethics approval may be obtained from an Internal Ethics Committee, provided regulatory requirements are met. Incorrect risk classification can lead to rejection during ethical review or when BPOM evaluates the DIP.
Risk Classification Overview
| Aspect | High-Risk Cosmetic Trial | Low-Risk Cosmetic Trial |
|---|---|---|
| Application Area | Sensitive areas (eyes, mucosa, genital area) | General skin areas |
| Ingredients | Nano materials, specific skin-whitening agents, untested ingredients | Ingredients with established safety data |
| Test Type | Irritation, sensitization, phototoxicity, SPF testing | Minimal-risk efficacy testing |
| Product Type | Anti-aging products, SPF products | Basic moisturizers, cleansers |
| Target Subjects | Vulnerable groups, long-term exposure | Healthy adults, short-term exposure |
Regulatory consequence:
- High-risk trials → Approval from an independent Ethics Committee is mandatory
- Low-risk trials → Ethics approval may be obtained from an Internal Ethics Committee, provided requirements are met
 Ethical Approval Must Be Obtained Before the Trial
Ethical Approval Must Be Obtained Before the Trial
The amended Article 6 and Article 7 clarify that:
- Ethical approval must be obtained before the clinical trial begins
- Informed Consent must be signed prior to any intervention
- No trial activity is allowed before ethics approval is issued
Conducting a trial first and requesting ethics approval afterward is strictly prohibited.
This protects:
- Subject rights
- Subject safety
- Data integrity and scientific validity
 How Clinical Trial Data Is Used
How Clinical Trial Data Is Used
Clinical trial results are used to support cosmetic claims, provide evidence of safety and benefits, and fulfill the Safety and Benefit section of the Product Information Document (DIP). The DIP is not uploaded during cosmetic notification, but it must be complete and ready in case of BPOM audit, product sampling, or post-market surveillance.
If clinical evidence or ethics documentation is missing or non-compliant, BPOM may question the claims, request revisions, or take regulatory action after notification approval.
The Simple Rule to Remember
Higher claim = higher responsibility
If your cosmetic claim is strong,
your scientific evidence must be just as strong.
Frequently Asked Questions (FAQ)
Q: Do all cosmetic products require BPOM Clinical Trial Approval?
A: No. For cosmetics, clinical trials are required when needed to substantiate safety or benefit claims, especially for high-risk products or innovative claims. However, cosmetic clinical trials conducted in Indonesia must still follow the ethical and procedural standards in this regulation.
Q: Our clinical trial was completed before 2025 without referring to Annex IIIa. Is it still valid?
A: It depends.
While the regulation is new, principles of scientific validity and research ethics have always applied. If the trial lacks proper methodology, ethics approval, or informed consent, BPOM may reject it as valid supporting evidence in the DIP. A regulatory review is strongly recommended.
Q: Our product already has a clinical trial from overseas. Can we use it?
A: Yes, sometimes.
Overseas clinical trial data may be used if the data is ethical, scientifically valid, and supports the same or lighter claims used in Indonesia.
How INSIGHTOF Can Help You
INSIGHTOF supports cosmetic registration in Indonesia by helping brands understand whether their product claims are already supported by the data they have.

Before you submit a cosmetic notification, we help you:
- Review your existing clinical trial data
- Check whether your claims match the available evidence
- Identify potential regulatory risks early
- Advise whether you need to adjust claims or prepare additional supporting data
This way, you can avoid unnecessary delays, claim revisions, or issues during BPOM audit and post-market surveillance.
If you are unsure whether your product data is sufficient, do not guess.
Let regulatory experts review it first—so you can register your product with confidence.
Regulatory compliance is not about guessing or assumptions. Each product must be assessed case by case based on claims, ingredients, and available data.








