Indonesia’s cosmetic regulatory landscape in 2025 is no longer about simply “having a license.” It is about surviving under tighter global scrutiny, stricter enforcement, and a regulator that increasingly sees itself as a peer to the world’s most advanced authorities. For local and foreign brands alike, compliance has shifted from a bureaucratic hurdle into a core business risk.
Drawing on years of regulatory practice and hundreds of brand registrations since 2016, INSIGHTOF Consulting Indonesia highlights ten developments that defined BPOM oversight of cosmetics in 2025.
Technical Overhaul Under BPOM Regulation No. 25 of 2025
One of the most significant regulatory milestones in 2025 is the enactment of BPOM Regulation No. 25 of 2025 on Technical Requirements for Cosmetic Ingredients. This regulation replaces previous standards and formally aligns Indonesia with the ASEAN Cosmetic Directive (ACD). A notable change includes the official terminology shift from “Kosmetika” to “Kosmetik” to simplify risk communication.
The regulation introduces a major update to ingredient classifications, including the addition of 101 substances to the prohibited list based on updated toxicological data.

BPOM Gains WHO Listed Authority Status, Raising Global Compliance Standards
Indonesia entered a new regulatory era after BPOM was officially recognised as a WHO Listed Authority (WLA). This status elevated BPOM into the ranks of globally trusted regulators, boosting the international credibility of BPOM-certified products. Yet the recognition came with consequences. Evaluation standards, inspections, and post-market surveillance are now expected to mirror international benchmarks. What once passed as “acceptable local practice” is increasingly treated as non-compliance.

Photo Sources: Biro Kerja Sama dan Hubungan Masyarakat BPOM
Misleading Claims in Men’s Cosmetics
In late 2025, BPOM revoked the licenses of 13 men’s cosmetic products promoted with exaggerated and implausible claims, including promises to improve sperm quality or cure impotence. The case underscored a shift in enforcement priorities. Safety alone is no longer sufficient. Marketing narratives, labels, and digital advertisements are now scrutinised as closely as formulations. For brand owners, creative marketing has become a regulatory liability.

Nationwide BPOM Operation Uncovers Rp31.7 Billion in Illegal Cosmetics
February 2025 marked one of BPOM’s most aggressive nationwide enforcement actions. Coordinated inspections across Indonesia uncovered illegal cosmetic production and distribution valued at Rp31.7 billion, nearly ten times the previous year’s figure. Most seized products lacked marketing authorisation; many contained prohibited substances. The majority were imported “viral” cosmetics sold online. The message was unmistakable: digital sales channels are no longer regulatory blind spots.

Photo Sources: Biro Kerja Sama dan Hubungan Masyarakat BPOM
Police and BPOM Raid Illegal Cosmetic Warehouse, Signalling Criminal Enforcement
Administrative sanctions are no longer the ceiling of enforcement. A joint operation between BPOM and the national police dismantled an illegal pharmaceutical and cosmetic warehouse in Jakarta worth Rp2.74 billion. The case demonstrated a growing willingness to pursue criminal charges against actors in the illegal supply chain. For distributors and importers, compliance failures increasingly carry legal—not just commercial—risk.

Photo Sources: Biro Kerja Sama dan Hubungan Masyarakat BPOM
Year-End Sales Trigger Intensified Monitoring of Offline and Online Markets
As year-end shopping peaks approached, BPOM intensified inspections during the November–December discount season. Almost half of inspected physical outlets failed compliance checks. Online findings were worse: more than three-quarters of reviewed sales links lacked valid marketing authorisation. The surge in violations revealed a structural problem—many brands treat compliance as a one-off approval, rather than a continuous obligation.
Offline: Out of 984 facilities, 48% were non-compliant. The highest violation was lacking a distribution permit (94.3%), followed by hazardous ingredients and expired goods.
Online: 5,313 problematic links were identified. 77% lacked distribution permits and 23% contained hazardous ingredients.

New Rule Requires Formal Risk Assessment of Cosmetic Raw Materials
The introduction of BPOM Regulation No. 26 of 2025 formalised mandatory risk assessments for raw materials used in cosmetics and related products. Prompted in part by past contamination scandals involving toxic solvents, the regulation forces manufacturers to justify material safety with scientific evidence. Ingredient sourcing has become a regulatory decision, not merely a procurement one.


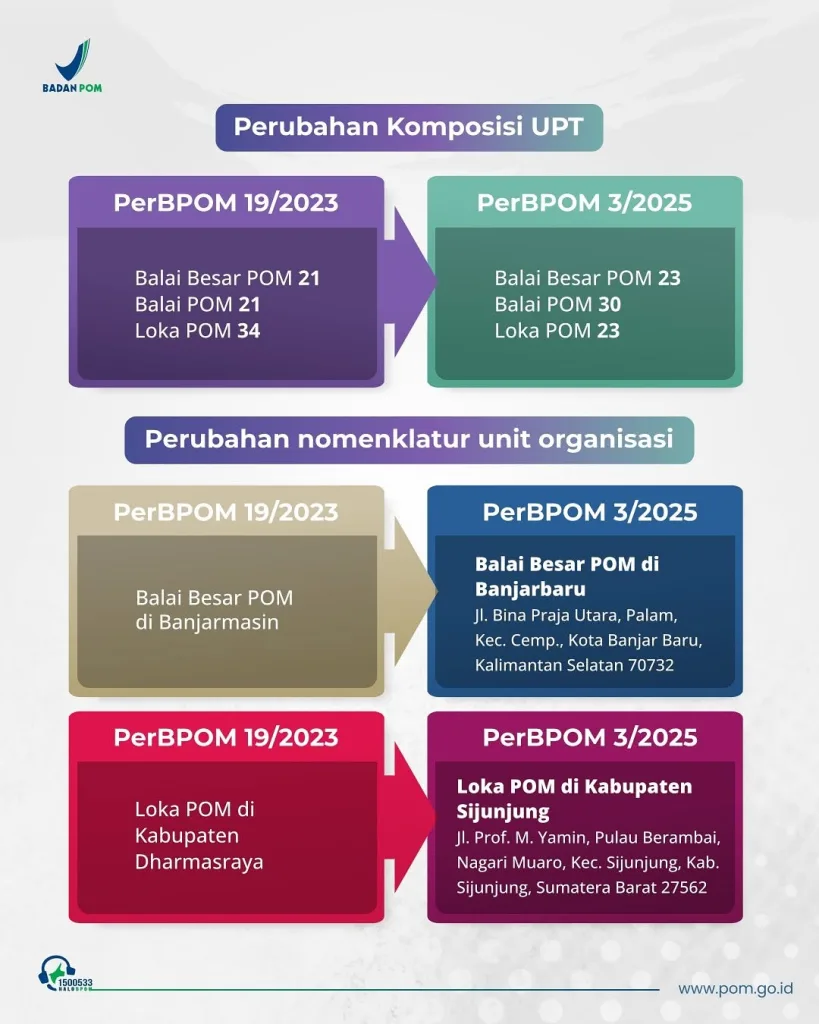
Reorganisation of Regional BPOM Offices Alters Inspection and Licensing Dynamics
Behind the scenes, BPOM also restructured its regional technical units under BPOM Regulation No. 3 of 2025, upgrading the classification of several offices. While largely administrative, the change affects inspection authority, service speed, and jurisdictional reach. For businesses, where a facility is located increasingly determines how—and how quickly—it is regulated.

The “Detective Doctor” Effect on Market Surveillance
Public scrutiny intensified through the rise of social media figures known as “Dokter Detektif” (Doktif), who conducted independent laboratory testing on viral cosmetic products. While this trend increased transparency, it also demonstrated that regulatory enforcement applies universally.
The revocation of products associated with Doktif herself—due to ingredient inconsistencies—underscored that compliance must be grounded in verifiable data, not online popularity.

Draft CPKB 2025 Signals Shift Toward Practical Manufacturing Compliance
BPOM’s draft 2025 Good Cosmetic Manufacturing Practice (CPKB) regulation signalled a quieter but significant reform. The proposed framework reduces redundant administrative requirements and shifts attention toward real-world quality systems. The reform aims to align Indonesian manufacturing standards more closely with international GMP expectations. For manufacturers, paperwork may ease—but inspection standards will not.

About INSIGHTOF Consulting Indonesia
INSIGHTOF Consulting Indonesia provides end-to-end regulatory affairs services for companies entering and operating in the Indonesian market. Our expertise covers BPOM and Ministry of Health (Kemenkes) registrations for cosmetics, medical devices, food, PKRT (household health supplies), health supplements, and Halal certification.
With in-depth knowledge of the latest 2025 regulatory developments, we support clients across the full compliance lifecycle—from dossier preparation and notification submission to post-market surveillance and audit readiness.

Conclusion
The year 2025 marks a decisive shift in BPOM’s regulatory approach—data-driven, technology-enabled, and uncompromising on integrity. The message is clear: safety and compliance are non-negotiable.
Brands that embed regulatory compliance into their core business strategy will not only survive but thrive in Indonesia’s competitive beauty market. INSIGHTOF Consulting Indonesia stands ready to support your journey toward sustainable and compliant growth.







