In general, a single product cannot be registered with BPOM (Indonesian FDA) by two different companies simultaneously if the product data is identical (composition, brand, packaging, etc.). Each BPOM Registration Number (NIE) is tied to one company that legally owns and is responsible for that specific product.

Basic Principles of Product Registration with BPOM
- One legal responsible party:
Every product registered for distribution in Indonesia must have a single, clearly designated legal entity responsible for it under Indonesian law. This entity, known as the Company Representative Office (CRO), is typically an Indonesian-based company – often your chosen local distributor or a subsidiary.
Foreign manufacturers cannot directly register their products with BPOM. They must appoint an Indonesian CRO. This makes selecting a trustworthy and capable local partner (distributor) absolutely vital, as they will be your official representative and legal arm in Indonesia. The relationship is formalized through an Appointment Letter or Power of Attorney, which is a key document for the registration process.

- Unique registration number:
The registration journey officially begins when your designated Indonesian CRO creates an account on the BPOM e-Registration system. This online portal is the central hub for all product registrations. Upon successful evaluation and approval, BPOM issues a unique Nomor Izin Edar (NIE), which translates to Distribution Permit Number.
The issued NIE will explicitly display the name of the applicant company (your Indonesian CRO/distributor). This reiterates that the local entity, not the foreign manufacturer, holds the direct registration. The NIE serves as public proof that the product has undergone BPOM’s rigorous assessment and is authorized for sale in the Indonesian market. It is also essential for customs clearance.
- No duplication allowed:
BPOM enforces a strict “no duplication” policy to maintain data integrity, prevent market confusion, and avoid legal disputes. This means that for a given product (defined by its formulation, packaging, and brand name), only one valid BPOM registration can exist at any one time.
If two different companies try to register the exact same product (with identical data), BPOM will reject one of them to prevent data duplication and legal disputes over product responsibility.

Exceptions & Important Notes
While BPOM maintains a strict “one product, one registration” policy, there are crucial exceptions and procedures to understand, particularly concerning variations and changes in distribution:
- Product data variations:
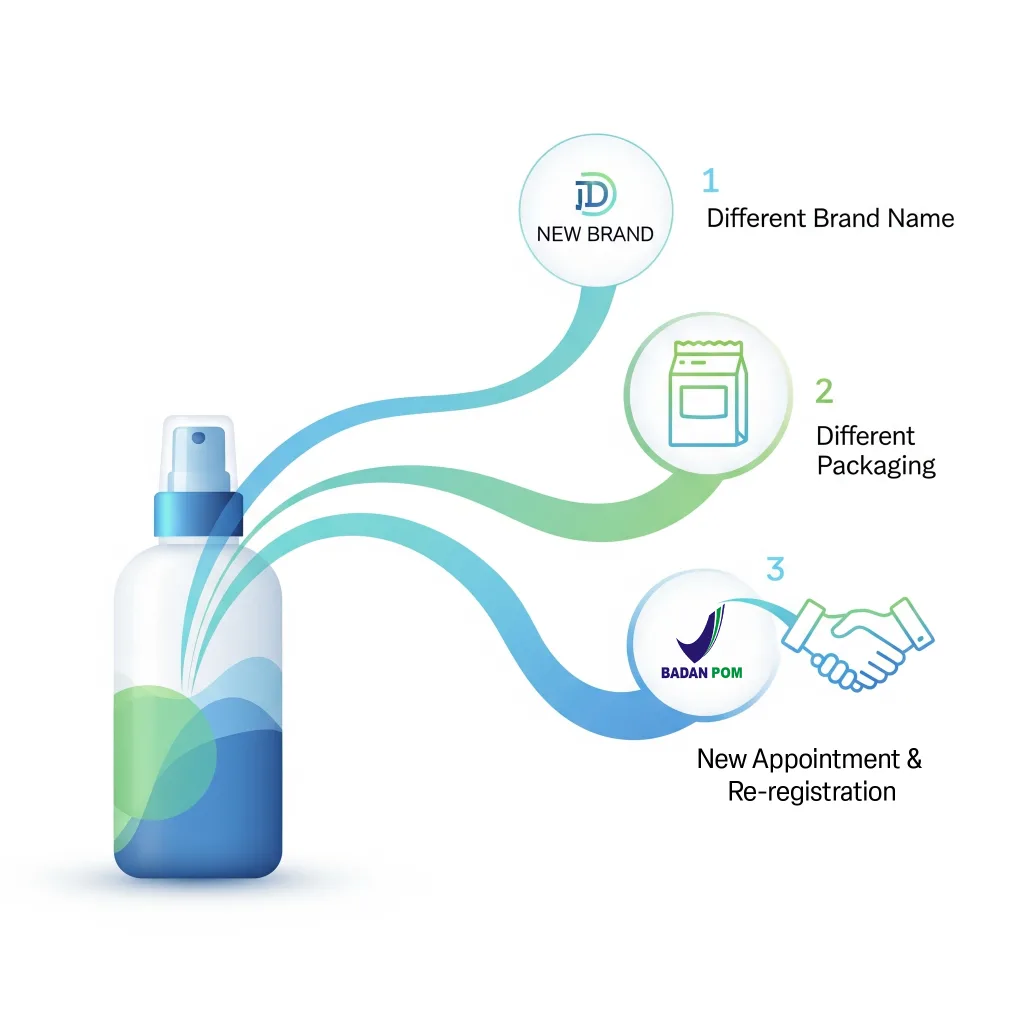
If there are genuinely significant differences in the product as defined by BPOM’s criteria, then multiple registrations are permissible, even if the core substance might share similarities. BPOM considers a product “different” if it has distinct:
- Brand Names: Products from the same manufacturer but marketed under different brand names (e.g., “XYZ Day Cream” vs. “ABC Brightening Cream”) are considered separate entities.
- Packaging: Even if the brand name is the same, vastly different primary or secondary packaging (e.g., a tub vs. a pump bottle, or different sizes/formats that impact usage or claims) can sometimes constitute a “different product” requiring a separate NIE.
- Formulation/Ingredients: Any significant alteration in the ingredient list, concentration of active ingredients, or intended use will definitively require a new registration. Minor changes to excipients might require notification rather than a full new registration, but this needs careful assessment.
- Imported products with different distributor
Multiple Indonesian companies may register products from the same foreign factory only if they each have a formal appointment letter from the manufacturer and submit different product data (e.g., different brand or packaging).
Crucially, each distributor must submit different product data. This usually means they are responsible for a distinct brand name or a unique line of products from that manufacturer. For instance, Manufacturer X produces “Brand A” and “Brand B.” Distributor P could be appointed for “Brand A,” and Distributor Q for “Brand B,” even though both brands come from Manufacturer X’s factory. They cannot both register the identical “Brand A” product.
- Change of Distributor Requires New Registration
If Company A no longer holds the distribution rights and the manufacturer decides to appoint Company B instead, this cannot be done through a license transfer or double registration.
Instead, the manufacturer must issue a new official appointment letter for Company B, and Company B must submit a brand-new BPOM registration using its own company account.
Legal Risks & Implications
- Application rejection: Duplicate registration attempts can lead to application rejection by BPOM.
- Trademark and license disputes: Without a clear agreement, multiple parties may dispute over brand rights or product ownership.
- Product accountability: Only the holder of the NIE is legally responsible for the product’s safety, quality, and information in the market.
Example Case: Cosmetic Product from Korea
Suppose Company A in Indonesia wants to register a Korean cosmetic product. But Company B is also interested in marketing the same product.
Since the Korean manufacturer can only appoint one official importer (usually through an exclusive agreement), then:
- Only the company with the official appointment letter from the manufacturer can register the product with BPOM.
- BPOM will reject any other company that submits a registration for the same product with identical data (same brand, same composition).
What If Company B Still Wants to Sell the Same Product?
1. Register a Product with a Different Name
Company B may collaborate with the same manufacturer to produce a similar product under a different brand name.
2. Act as a Sub-Distributor
Company B may collaborate with Company A (the license holder) as a sub-distributor. In this case:
- Company A retains the NIE and full legal responsibility.
- Company B acts only as a marketing or distribution partner.
Conclusion
- One identical product can only be registered by one company with BPOM.
- If two companies wish to register the same product, there must be variations in the product data, or the license must be transferred legally.
Tips for Foreign Distributors
- Appoint one official importer in Indonesia to handle BPOM registration.
- Establish a clear exclusive distribution agreement to avoid future disputes.
- Check product registration status on BPOM’s official site: cekbpom.pom.go.id
At INSIGHTOF, we help you navigate manufacturer registration and every step of the cosmetic approval process, so you can bring your products to market faster and hassle-free. Ready to streamline your Indonesia launch? Let’s chat!
Do you need assistance registering your product in Indonesia?
Contact us today to start your registration process.








